Pharmaceutical Pipeline of Indication Specific Products
Developed Through the Company’s R&D, Clinical and Commercial Platforms
Pharmaceutical Pipeline
Indication-Specific Products
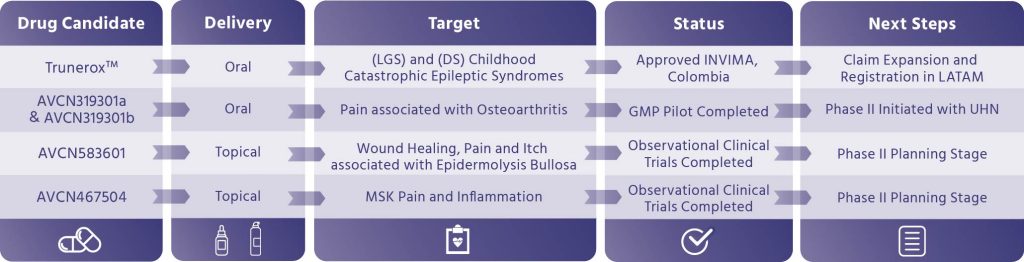
Trunerox™ 10% CBD Oral Solution
Adjunctive Treatment Of Seizures Associated with Lennox Gastaut Syndrome (LGS) and Dravet Syndrome (DS)
Trunerox™ is a pharmaceutical drug with proprietary formulation manufactured under GMP standards and utilizing the Company’s purified API. Clinical indications show Trunerox™ shows potential as an adjunctive treatment for seizures associated with Lennox Gastaut Syndrome (LGS) and Dravet Syndrome (DS).
Marketing authorization has been obtained in Colombia by INVIMA in Q1, 2024. Commercialization in Colombia is expected to take place in 2025.
Pharmaceutical Pipeline and Drug Candidates
Designed to Address 5 Major Clinical Areas
Leveraging the company’s scientific platform and vertical integration to deliver proprietary and accessible finished products, these cannabinoid-based drug candidates are designed to address unmet medical needs in the areas of dermatology, chronic pain, and various neurological and mental conditions. Candidates are in various stages of R&D, pre-clinical to real world evidence studies and or registration stage.