Avicanna Announces Pre-Clinical Data Supporting Enhanced Absorption and Patent Filing for Novel Oral Delivery Platform
PwdRx novel drug delivery platform demonstrated superior bioavailability and accelerated absorption of cannabinoids in preclinical studies
Provisional patent application on the novel drug delivery platform which can be loaded into products such as tablets, capsules, sachets and pouches
TORONTO, November 19, 2025 – Avicanna Inc. (“Avicanna” or the “Company”) (TSX: AVCN) (OTCQX: AVCNF) (FSE: 0NN), a biopharmaceutical company focused on the development, manufacturing, and commercialization of plant-derived cannabinoid-based pharmaceuticals, today announced positive preclinical results and the filing of a provisional patent application with the United States Patent and Trademark Office (USPTO) for its novel Powder Drug Delivery System (“PwdRx”) platform.
The drug delivery platform has been specifically developed to address key formulation challenges associated with highly lipophilic cannabinoids (CBD, CBG, THC and CBN), which often exhibit poor water solubility, low and variable bioavailability, and delayed onset of action. In preclinical pharmacokinetic studies, the PwdRx formulations demonstrated enhanced bioavailability and faster uptake compared to conventional medium-chain triglyceride (MCT) oil-based formulations.
Recent in-vitro study of Avicanna’s PwdRx technology demonstrated statistically significant results including 74% higher bioavailability (AUC), 63% faster peak plasma levels (TMAX) and a 134% higher peak plasma concentration (CMAX) when compared to MCT oil formulation.
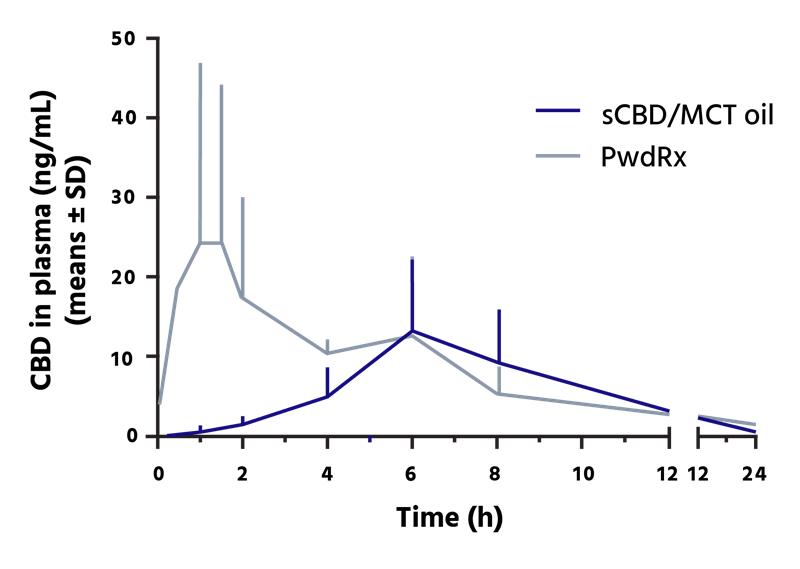
In addition to enhanced bioavailability and faster uptake, the technology is also designed to deliver long-term stability and manufacturing versatility, including supporting scalable pharmaceutical production in a range of solid-oral and powder-based formats, including tablets, capsules, sachets, and pouches. The pharmaceutical dosage form is also designed to offer tunable drug release capabilities to enable differentiated product profiles to further enable tailoring to specific patient needs
“By improving dispersion, solubility and consistency of uptake, the PwdRx platform has the potential to enable health care providers greater flexibility to tailor potential treatment options for patients managing conditions associated with pain and inflammation,” said Dr. Karolina Urban, EVP, Medical and Scientific Affairs of Avicanna. “We are encouraged by the data and look forward to further advancing the technology into our pharmaceutical pipeline and for further clinical evaluation.”
The provisional patent filing covers the composition, methods of use, and potential utility of the PwdRx platform in pain and inflammation, including conditions such as rheumatoid arthritis, osteoarthritis, neuropathic pain, fibromyalgia, migraine, and multiple sclerosis.
“The advancement of our PwdRx platform represents another significant step forward in the science and technology of cannabinoid delivery and further strengthens our global intellectual property portfolio,” said Aras Azadian, Chief Executive Officer of Avicanna. “We believe this novel technology will unlock potential new and different pharmaceutical and commercial product opportunities and further demonstrates Avicanna’s leadership in advancing innovation in the science and technology of cannabinoids.
About Avicanna:
Avicanna is a commercial-stage international biopharmaceutical company focused on the advancement and commercialization of cannabinoid-based products and formulations for the global medical and pharmaceutical market segments. Avicanna has an established scientific platform including R&D and clinical development leading to the commercialization of more than thirty proprietary, evidence-based finished products and supporting four commercial stage business pillars.
- Medical Cannabis formulary (RHO Phyto™): The formulary offers a diverse range of proprietary products including oral, sublingual, topical, and transdermal deliveries with varying ratios of cannabinoids, supported by ongoing patient and medical community education. RHO Phyto™ is an established brand in Canada currently available nationwide across several channels and expanding into new international markets.
- Medical cannabis care platform (MyMedi.ca): MyMedi.ca is a medical cannabis care platform formed with the aim to better serve medical cannabis patients’ needs and enhance the medical cannabis patients’ journey. MyMedi.ca is operated by Northern Green Canada Inc. and features a diverse portfolio of products and bilingual pharmacist-led patient support programs. MyMedi.ca also provides specialty services to distinct patient groups such as veterans and collaborates with public and private payers for adjudication and reimbursement. MyMedi.ca provides educational resources to the medical community to facilitate the incorporation of medical cannabis into health care regimens.
- Pharmaceutical pipeline: Leveraging Avicanna’s scientific platform, vertical integration, and real-world evidence, Avicanna has developed a pipeline of proprietary, indication-specific cannabinoid-based candidates that are in various stages of clinical development. These cannabinoid-based candidates aim to address unmet needs in the areas of dermatology, chronic pain, and various neurological disorders.
- Active pharmaceutical ingredients (Aureus Santa Marta™): Active pharmaceutical ingredients supplied by the Company’s majority owned subsidiary Santa Marta Golden Hemp SAS (“SMGH”) is a commercial-stage business dedicated to providing various forms of high-quality CBD, THC and CBG to the Company’s international partners for use in the development and production of food, cosmetics, medical, and pharmaceutical products. SMGH also forms part of the Company’s supply chain and is a source of reliable input products for its consumer retail, medical cannabis, and pharmaceutical products globally.
SOURCE Avicanna Inc.
Stay Connected
For more information about Avicanna, visit our website or contact Ivana Maric by email at info@avicanna.com.
Cautionary Note Regarding Forward-Looking Information and Statements
This news release contains “forward-looking information” within the meaning of applicable securities laws. Forward-looking information contained in this news release may be identified by the use of words such as, “may”, “would”, “could”, “will”, “likely”, “expect”, “anticipate”, “believe”, “intend”, “plan”, “forecast”, “project”, “estimate”, “outlook” and other similar expressions. Forward-looking information contained in this news release includes, without limitation, statements related to the Company’s future business operations, the opinions or beliefs of management and future business goals. Although the Company believes that the expectations and assumptions on which such forward looking information is based are reasonable, undue reliance should not be placed on the forward-looking information because the Company can give no assurance that they will prove to be correct. Actual results and developments may differ materially from those contemplated by these statements. Forward-looking information is subject to a variety of risks and uncertainties that could cause actual events or results to differ materially from those projected in the forward-looking information. Such risks and uncertainties include but are not limited to current and future market conditions, including the market price of the common shares of the Company, and the risk factors set out in the Company’s annual information form dated April 11, 2025, filed with the Canadian securities regulators and available under the Company’s profile on SEDAR+ at www.sedarplus.ca. The statements in this news release are made as of the date of this release. The Company disclaims any intent or obligation to update any forward-looking information, whether as a result of new information, future events or results or otherwise, other than as required by applicable securities laws.



